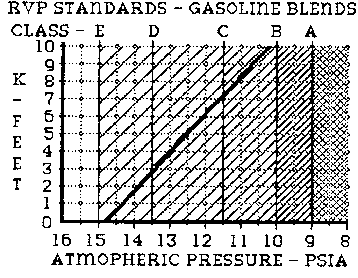
 CLASS C: RVP of 11.5
psia. Summer. Areas including eastern US and eastern Washington and
Oregon.
CLASS C: RVP of 11.5
psia. Summer. Areas including eastern US and eastern Washington and
Oregon.| FOR THE LACK OF LEAD AND OCTANE |
By Kiyoshi Hamai
Chapman Report - November 1987
This is a series of articles concerning the liquid stuff that we continually pour gallons upon gallons into our petrol tanks, only to have it vaporized and pumped into the atmosphere as good old smog. In this series we will consider petrol topics such as RVP i (Reid Vapor Pressure), Octane content and boosters, and Valve Recession and protection.
In this first installment we will consider Reid Vapor Pressure (RVP). RVP is not the most frequently thought of aspect of petrol. In fact most of the C/R readers probably have never heard of RVP and yet it perhaps contributes more to the performance of your Lotus than the lead and octane content of the petrol in your tank.
First some basics. The explosion that takes place within the confines of the cylinder of your engine is merely extremely rapid oxidation or, burning. Like any fire or oxidation two very important elements are it required. 1) Fuel and; 2) Oxygen (in gaseous form). Ask any fireman how to extinguish a fire and he'll explain how to eliminate the fuel (backfire used in fighting forest fires) or cut off the oxygen supply (smothering it with dirt, blankets or water).
Therefore to enhance the burning chemical reaction the idea is to supply both fuel and oxygen in ample quantities. This confirms the fact that fuel while in its liquid form will not ignite. That in fact it is the fuel vapor that burns. The operative word here is vapor. Vapor is the "gaseous" form of the liquid fuel. This mixed with oxygen creates a situation where burning can take place. It follows then that the ease with which fuel changes from liquid to gas, or its volatility has direct effect upon the efficiency of the combustion. The measurement used to express fuel's volatility is expressed in term of Reid Vapor Pressure (RVP). RVP is calculated to reflect the vapor pressure at 100 degrees F. Vapor pressure can be thought of as the pressure exerted by the molecules of fuel escaping in the atmosphere.
A couple of laws of physics come into play. First, as temperature increases the molecules in the fuel become more excited and can more easily escape into the atmosphere. A pot of boiling water is perfect example of extremely excited molecules of water racing to escape into the atmosphere. The hotter the water becomes therefore, the higher the vapor pressure, until finally the vapor pressure equals the atmospheric pressure. At this point the liquid is free to become vapor without additional heat (boiling).
Secondly, the atmospheric pressure affects the ease with which the fuel molecules vaporize. Think of the atmospheric pressure as weight keeping the liquid from vaporizing. Therefore it would follow that as atmospheric pressure drops that the liquid would more easily vaporize.
The combination of low atmospheric pressure and high temperature makes fuel evaporation a certainty. As temperature rises and atmospheric pressure drops, the question of the fuel vaporization is not "if" but clearly "When" the vapor pressure will overcome the atmospheric pressure. Hence fuels with high RVPs will vaporize more easily.
One would unthinkingly assume that a high RVP would be desirable, concluding that with a high RVP the fuel could be properly vaporized with air in the carbs or injectors ports. That's true except that it is possible for fuels to have RVP numbers high enough that they will vaporize while still in the fuel tank or in the fuel lines creating a situation commonly known as dreaded vapor lock.
Over the recent decade with the reduction of lead and greater demands place upon gasolines the RVP numbers have gradually crept upward and yet there are no standards by which gasolines must ;be refined with respect to RVP. Refiners are free to choose whether or not they voluntarily adhere to the RVP recommendations of the American Society for Testing Materials (ASTM).
The ASTM has divided the US into districts and issued fuel volatility recommendations based upon altitudes and seasonal characteristics. Summer blends therefore have to have lower RVP numbers than winter blends (summer being generally warmer then winter). The ASTM standards allow summer blends to have RVP as high as 11.5 psia (pounds per square inch absolute), and winter blends to 15 psia.
Consider the following normal atmospheric pressure at sea level is 14.69 psia. As we climb into high elevations the pressure drops to about 12 psia at 6000 feet and can dip below 10 psia at 11,000 feet. If the temperature were under 100 degrees F climbing that hill could be done. But, say you had a load of winter fuel, RVP 15 psia, and headed up into the mountains where you encountered 12 psia and 68 degree temps. What would happen? Don't worry, you'd never make there, since long before the fuel would have boiled in your fuel lines and you'd have vapor lock.
Aa you can see there are five classes of RVP fuel specification recommended by ASTM, classes A to E. Classes D and E are winter blends and A, B and C summer. ASTM recommends the following:
CLASS A: RVP of 9 psia. Summer. Areas including southern Nevada. Arizona, New Mexico and Mohave Desert of California.
CLASS B: RVP of 10 psia. Summer. Areas including most of the Western US (west of Mississippi) with the exception of Western Washington and Oregon.
 CLASS C: RVP of 11.5
psia. Summer. Areas including eastern US and eastern Washington and
Oregon.
CLASS C: RVP of 11.5
psia. Summer. Areas including eastern US and eastern Washington and
Oregon.
CLASS D: RVP of 13.5 psia. Winter. Areas including southern California, southern Nevada, Arizona, New Mexico, Texas, Louisiana, Mississippi, Alabama, Georgia, South Carolina, and Florida.
CLASS E: RVP of 15 psia. Winter. Areas including Oregon Washington, Idaho, Montana, Wyoming, Northern Nevada, Utah, Colorado, North and South Dakota, Nebraska, Kansas, Wisconsin, Iowa, Missouri, Minnesota, Illinois, Indiana, Ohio, Kentucky, Michigan, West Virginia, Virginia, Pennsylvania, DC, New York, Maryland, Connecticut, Rhode Island, New Jersey, Vermont, New Hampshire, Maine and Massachusetts.
Additionally, certain areas can either have Class D or E fuels in the winter. They are Northern California, upper most New Mexico, Oklahoma, Arkansas, Tennessee and North Carolina. The effects of all this are ten fold. We, as Lotus owners who often rarely drive our cars in the winters may have trouble in the spring after filling up in the winter. Next, it could be of some benefit in performance to blend winter fuel with summer fuel on cooler spring days.
So, now we understand how the volatility of the fuel in your gas tank, as measured as RVP (Reid Vapor Pressure). Recall a high RVP number represents a fuel that vaporizes more easily.
Thus a fuel with high RVP number would allow a vehicle to start more easily on cold mornings, yet such a fuel may, on warm days vaporize in the fuel lines /or fuel pump causing vapor lock.
Now, we consider octane, simply put a number which describes a fuels ability to eliminate knock and ping. The higher the octane number the greater the fuel's anti-knock capability. Therefore octane has no relationship to exhaust valve recession, or the fuel's ability to vaporize or even the power available.
Aside, the power available corresponds more closely to the BTU rating or the specific gravity, which is the ratio of the density of the fuel compared to water. It is assumed the greater the density the more BTU's are packed into the fuel. A wonderful example of this is the weight of F1 petrol compared to pump fuel. The F1 stuff is considerably heavier, and in fact the fuel used in the Turbo engines is heavier than the petrol used in the normally aspirated cars.
So, on with octane. Among the different ways to measure octane are Research Octane numbers, which are usually artificially high and only indicate real world 'tip-in" ping tendencies. The other method is called Motor Octane, which is more representative of real-world full throttle performance. Today the octane numbers posted at the pump is the average of these two: (R+M)/2. Old timers will recall those days of 104 octane at the pump, "Good ol' days...real gas...", they claim. But, the honest truth is those old octane numbers were Research Octane numbers, which is better represented as the best octane number, that could be attained by a fuel. These conditions might be best represented by light loads and light throttle (near idle!).
So, why are we so concerned about octane? Simple, since the octane number represents the resistance of the fuel to knock it can be said the octane number measures the resistance of the petrol to detonate on its own when compressed. This is a lesson in thermodynamic physics, where we learn that when a set volume of a gas is compressed it heats up. Conversely, an expanding volume of gas cools, explaining the condensation that forms around a propane bottle on a camp stove. Thus, as the air fuel mixture is compressed within a cylinder it is heated both by the work performed upon it and by the warm cylinder walls. The more volatile the fuel the lower the octane) the more susceptible it is to exploding on its own accord (ie. the knocking or pinging sound is the noise of the explosion taking place prior to full compression).
Now, considering that the efficiency of an internal combustion engine is closely related to the timing of the explosion in the cylinder, one can easily relate that if one is unable to control the precise moment of the explosion that the engine will not run at its peak efficiency. Thus we would not maximize the conversion of the energy in the fuel into mechanical energy.
The most widely used fuel additive to alter octane number until recently has been Tetraethyl Lead. And, as of today this additive is unsurpassed in it ability to increase octane numbers. Unfortunately this additive has the undesirable effect of polluting the air with lead oxides which eventually are cycled into the food cycle are digested by animals and humans. The effect of lead within humans and animals has been well documented are extremely unpleasant. Therefore today all pump gas is lead-free and other additives are used to increase octane numbers. These additives will be discussed in the next installment, but for this segment we will concentrate on fuels using Tetra-ethyl Lead.
Contrary to what many believe there are sources of lead gas, namely AV-Gas and Racing Gas. Both, it should be stated are illegal for street use, (However, they may be technically legal if used as additives to pump gas) because neither have highway taxes added and have tetra-ethyl lead contents far far exceeding the limits. This author and this publication in no way recommends the use of AV-Gas or Racing Gas for street use, but what follows is a discussion of past uses and experiences with these in automotive engines.
Blending Aviation and Race Gas with Pump Premium Unleaded Gasoline
|
Blend |
RON |
MON |
(R+M)/2 |
Specific Gravity |
Approx Lead Content Gm/Gal |
|
|
100/130 AVGas |
Unleaded Premium 75%/25% 50%/50% 25%/75% 100/130 AVGas |
96.8 99.2 100.9 101.3 101.5 |
85.8 90.3 94.4 99.8 103.7 |
91.3 94.8 97.6 100.6 102.6 |
0.7579 0.7416 0.7256 0.7093 0.6926 |
0 1 2 3 4 |
|
National Distributed Race Gas |
Unleaded Premium 75%/25% 50%/50% 25%/75% Race Gas |
96.8 101.8 104.9 109.2 114.2 |
85.8 91.1 95.1 100.1 103.5 |
91.3 96.4 100.0 104.6 108.8 |
0.7579 0.7547 0.7491 0.7440 0.7408 |
0 1 2 3 4 |
AV-Gas is relatively inexpensive, being regulated by the US Government and is absolutely consistent from batch to batch (regulated by the FAA). 100/130 AV-Gas has about the same Motor Octane Number (MON) as typical race gas. But, AV-Gas has a lower specific gravity and thus will require a richened carb and will result in lower mileage numbers than say race gas. AV-Gas is available at airports; buy it by the 55 gallon drum "for off highway use". Stay away from the inferior 80/87 AV-Gas. "Low " lead 100 AVGas has plenty of lead for any "street" car, but in blend has less octane boosting ability than 115/130. If you've got access to what's used by the Air Force or Navy you may have access to 115/145... that's the best gas in the world...
Race gas has a slightly higher energy content than AV-Gas, and thus offers a performance advantage in all-out competition.
One might get the impression that running straight Race Gas or AVGas would be the trick, but, no. The octane numbers are so high that these fuels will often continue burning all the way out the exhaust ports and will raise hell with the exhaust valves... melting them down. One might also consider obtaining Tetra-ethyl Lead (TEL) and adding it to your pump gas. First, you'd find obtaining TEL near impossible. And secondly, TEL is extremely dangerous, any skin contact in high concentration is sure DEATH!
So, another alternative is to blend Unleaded Premium pump gas with AVGas or Race Gas and the result maybe a usable fuel mixture. Figure 1 is a table illustrating some results of blending. Again you are warned that blending gasolines is dangerous and the storage of these liquids can endanger your home and your life! This is serious stuff and not recommended.
As previously stated the best octane booster known to man at this time is Tetra Ethyl Lead. The problem is two-fold: First, it is near impossible for the layman to obtain and second, it is so toxic in concentrated forms that is fatal to the touch! So, this month we'll discuss alternative octane boosters, some of which are sold "over" the counter at speed shops.
Taking them in alphabetical order the first is...
ANILINEAniline is highly effective octane booster, once widely used as the main ingredient in several aftermarket retail level canned additive products. Today, however Aniline has been phased out of most products of this type due to its undesirable characteristics. One can expect about one octane increase for every percent aniline added to the gasoline base stock, with a ceiling of 5% concentration. Aniline is an incredibly good solvent, removing combustion chamber deposits, thus raising the apparent octane. It'll eat through paint and damage any rubber (seals, hoses, diaphragms, etc). Aniline is EXTREMELY toxic; it can be absorbed through the skin, enter the blood stream, and starve the body of oxygen. It can not be shipped via UPS and a DEA permit is required to buy the stuff in bulk!
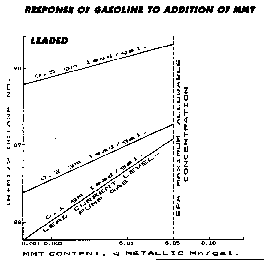
MMT
Commonly known as Methyl Cyclopentadienyl Manganese Tricarbonyl (if though you didn't know...). MMT was once hailed as the replacement for TEL (Tetra-Ethyl Lead), since it both prevents exhaust valve seat recession and raises octane (but, not as well as TEL). But, the EPA claims it fouls Catalytic Converters and increases Hydrocarbon emissions. Therefore the EPA has ruled that it can only be used in pre-'75 cars in concentrations that do not exceed 0.1g/gal. However, it is the principal component in several highly effective and promoted aftermarket retail level canned additives (including Moroso Octane Booster and Octane Boost Corp's 104+). Be aware that in concentrations exceeding 0.2 g/gal, MMT produces a hard metal compound that may erode the engine! MMT also leaves a "rusty" residue in the combustion chamber and spark plugs. So, don't panic when you pull your plugs after using such products.
OXYGENTATESThis is a large category of chemicals that actually participant in the combustion process. MMT and TEL do not actually participant in the combustion process, but do protect the exhaust valve seats. The various Oxygenates, alone and as blends raise octane and do participate in the actual combustion process, however they do not assist in prolonging valve seat life. The major forms of Oxygenates include the various alcohol compounds, MTBE (methyl tertiary butyl ether), nitrous oxide, and nitroparaffins.
The specific gravity of oxygentates is generally greater than gasoline, but this is misleading since this is mostly due to the added weight supplied by the "heavy" oxygen molecules. Thus the net effect of using an oxygenate is to lean out the overall air/fuel mixture. Thus is using high concentrations of oxygenates may require major fuel metering modifications.
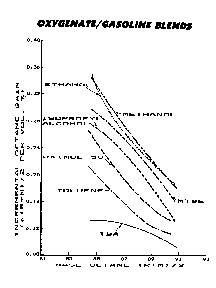
The alcohol compounds include ethanol, methanol, isopropyl and tertiary butyl alcohols. Commonly ethanol is the drinking stuff, isopropyl is the drug store variety. They are all relatively inexpensive, and provide a big bang for the octane buck, in pure form can boost octane as much 10%. The negatives are the weird side effects that are associated with these fuels.
Methanol is the most common alcohol octane booster, but creates the most severe side effects. Methanol can "absorb" water but, will deteriorate rubber (seals, hoses, diaphragms, etc), plastics, white metals (aluminums; carb bodies, heads, fuel pump bodies, etc), and fuel tank linings.
Ethanol is as effective as methanol but, its negative reactions are less severe, although ethanol will cause water to separate out to a degree.
All alcohols in pure forms have low RVP numbers, thus causing terrible cold start problems. For example if fueled by pure methanol an engine will not start below 60 degree F. However, when blended with gasoline both methanol and ethanol can raise the RVP number as much as 3 and 1 psi respectively, maximum effect occurring at 3% concentration. Other alcohols will lower the overall RVP. However the overall effect of using any blend is an increase in cold-start problems due the cooling effect caused by alcohol's high latent heats of evaporation. Consider too, that extreme alcohol concentrations will strip lubricants from the upper cylinder walls, inhibiting proper upper engine lubrication.
For short term use ethanol and methanol blends can assist in cleaning up tailpipe emissions (something to remember next registration...). On the long term alcohol blend use should be limited to about 10% concentration.
Gas/Alcohol Octane Ratings
|
Unleaded Regular |
Unleaded Premium |
|||||
|
RON |
MON |
(R+M)/2 |
RON |
MON |
(R+M)/2 |
|
|
Base Gas |
91.0 |
83.1 |
87.1 |
97.7 |
86.5 |
92.1 |
|
Base + 10% Methanol |
93.9 |
84.5 |
89.2 |
100.2 |
88.1 |
94.2 |
|
Base + 10% Ethanol |
94.9 |
84.7 |
89.8 |
100.7 |
88.4 |
94.6 |
MTBE, methyl tertiary butyl ether, in pure form ether has a terrible octane number, however MTBE is a fair octane booster in concentrations up to 10%. MTBE has no affinity for water and needs to be used in relatively high percentages to significantly boost octane. MTBE is the most popular additive of the major gas producers and is used in "clean" burn pump gas. Recently, there are major claims and studies to understand how quickly MTBE gets into ground water and its affect on drinking water.
Nitrous Oxide, this is the stuff that the street guys holler about, the same stuff that the dentist blows down your throat to make you "relax". It's known for its tremendous power increases. It requires a separate storage bottle and fuel augmentation system. It is used only when needed, remember the big boost button? Nitrous Oxide does not boost octane, rather increase power by adding oxygen molecules to the burn mixture and has excellent latent heat of evaporation, imparting dramatic cooling effects on the air/fuel charge.
Nitroparaffins (nitropropane, propylene oxide, peric acid, acetone) can also produce octane increases and power gains, but when used in high concentrations will severely lean out the engine. Propylene oxide will produce about a 3% power gain at a 5% concentration.
I really don't how much more there's to write about gasoline and additives, but it seems the more I write the more there seems to write about. So, on that optimistic note we'll forge into this. We'll cover a summary of some aftermarket gas additive products and a summary of some of the common additives that were discussed last month
This first table is a neat little summation of the common gas additive catagories.
Aftermarket Gasoline Additives
|
Product |
Manufacturer |
Claimed Use |
|
QX-500 Fuel Lubricant |
Champion Lubricants Inc 605 Laguna Richardson, TX 75080 |
Lead substitute, reduces apparent octane requirement |
|
STP Lead Substitute |
First Brands Corp 39 Old Ridgebury Rd Danbury, CT 06817 |
Lead substitute |
|
Quantum OI Quantum VS Quantum VS+01 |
Gold Eagle Co 4400 South Kildare Chicago, IL 60632 |
Octane Booster Valve wear preventative Both of the above |
|
Octane Booster Octane Booster #2 |
Moroso Performance Prod. 80 Carter Dr Guilford, CT 06437 |
Octane Booster (race) Octane Booster (street) |
|
104+ Octane Boost Super 104+ 104+ Real Lead |
Octane Boost Corp PO Box 271148 Dallas, TX 75227 |
Octane Booster Octane Boost, Hi-Concentrate Valve seat protectant |
|
No-Knox "G" No-Knox "T" |
Petrofoam International Inc PO Box 771447 Houston, TX 77215 |
Octane Booster, cleaner Above for turbocharged engines |
|
Dur-Alt |
Polar Molecular Corp 4901 Towne Centre #303 Sagniaw, MI 48604 |
Lead Substitute, lubricant, cleaner, corrosion inhibitor |
|
Gunk Lead Substitute Gunk Octane Performance Booster |
Radiator Specialty Co. PO Box 34689 Charlotte, NC 28234 |
Valve wear protectant, Deposit cleaner, raises apparent octane |
|
Sim-U-Lead |
Sta-Lube Inc. 3039 Ana Street Compton, CA 90224 |
Lead Substitute, cleaner |
|
TK-7S Super Octane Power Booster TK-7R "Big Daddy" |
TK-7 Corp 1300 NE 4th St. Oklahoma City, OK 73117 |
Lead substitute, fuel system cleaner
Above for racing |
|
Valve Saver |
Torco USA Lubricants Corp 12247 Lakeland Rd Santa Fe Springs, CA 90670 |
Lead substitute, cleaner |
|
Unocal 76 Valve Saver |
Unocal Corp PO Box 7600 Los Angeles, CA 90051 |
Valve wear protectant, fuel system cleaner |
|
C-5 Fuel Additives |
VP Hydrocarbons 20846 Lamm Rd Elmendorf, TX 78112 |
Octane booster, lead substitute, valve protector. |
This second table is a listing of a few of the gasoline additives that can be on the shelves of your local auto parts store or speed shop (or even major mass merchants, Longs, Target etc...).
Who's Who in Gasoline Additives
|
ADDITIVE |
ADVANTAGES |
DISADVANTAGES |
|
ALCOHOL - Methanol, ethanol, isopropyl alcahol, tertieary butyl alcohol |
Excellent octane boosting compounds; Oxygen bearing compounds, offers potential power gains; Relatively inexpensive; Reduces exhaust emissions (hydrocarbons & CO); Widely available commercially. |
Poor fuel mileage; Causes water to separate from gasoline (methanol absorbs water); Methanol is highly corrosive, "eats" rubber, aluminum, steel, plastic; Poor low temperature driveability; Leans mixture; Strips oil from cylinder walls. |
|
ANALINE |
Very good octane booster; Increases "apparent" octane rating by cleaning deposits in cylinder |
Highly toxic, absorbed through skin; Eats rubber, plastic, paint; expensive; hard to obtain. |
|
HIGH-OCTANE HYDROCARBONS (benzene, toluene, xylene, triptane, etc.) |
High-octane chemical gasoline molecule compounds; Makes fuel more dense, increasing mileage potential; Fully blendable with gasoline; Non-corrosive when mixed with gasoline |
Exists in unleaded gas, adding more may have no effect; Need more than 10% to be effective; Low RVP |
|
AVIATION GAS 100/130 |
High lead content, extremely effective as octane booster and valve protectant; Relatively inexpensive, wide available at airports, absolute consistent mix |
Illegal for street use; Some airports will not sell; Less dense than race or pump gas; Leans mixture; Latent heat of evaporation is lower than race gas; Lower RVP; Lower mileage. |
|
MMT (methyl cyclopentadienyl manganese tricarbonyl) |
Very good octane improver, less toxic than analine; Valve protectant; Available at retail outlets. |
Plugs catalytic converters; More than 0.2g/gal creates hard metal compound that erodes engine; Increase emissions; Leaves rusty coating on plugs. |
|
MTBE (methyl tertiary butyl ether) |
Fair octane improver up to 10% mix; No affinity for water. |
High percentage needed to raise octane; Fairly expensive |
|
NITROPARAFFINS (nitropropane, propylene oxide, peric acid, acetone, etc.) |
Oxygen and nitrogen bearing compounds raise octane and add more power. |
Leans mixture, negative effects of octane boosting properties; Corrosive to rubber & plastic. |
|
ORGANO-METAL LUBRICANTS |
Prevents exhaust valve recession |
Does not improve octane; Not EPA approved for unleaded gasoline. |
|
POLAR-ORGANIC LUBRICANTS |
Prevents exhaust valve recession |
Does not improve octane |
|
AMINE DETERGENTS |
Combustion chamber cleaners; carburetor cleaners; Mild anti-rust and anti-clog properties |
May increase induction system deposits, in higher concentrations; Does not improve octane; cleans fuel system in low concentrations |
|
POLYMERIC DISPERSANTS |
Fuel system cleaners; Stable at high temperatures |
Does not improve octane |
|
TETRAETHYL LEAD |
The most effective octane booster and valve protectant |
POISON - Completely phased out by EPA; not available to public in effective octane boosting concentrations; causes increased lifter, piston and bore wear; Causes exhaust system corrosion; not compatible with catalytic convertors. |
|
RACING GASOLINE |
High lead content; Specially formulated for cars; Better mileage than AVGas; Good latent heat of evaporation, cools air/fuel charge. |
Expensive; Limited availability; May be inconsistent from batch to batch; Lower RVP. |
|
NITROUS OXIDE |
Oxygen bearing, offers power increases; Activated only when needed; Excellent latent heat of evaporation; Widely available. |
Not an octane booster; not a valve protectant; Requires separate fuel system and storage bottle; Bottles must be refilled by dealer. |
|
WATER (injected) |
Suppresses detonation by cooling air/fuel charge; Injection system activated only as needed; Water is cheap. |
Does not support combustion, may slightly reduce power; Excessive water kills combustion; require separate container. |
Hey, if all else fails.... eat BEANS!... F*RT IN A JAR = pure Methane!
I hope this is the final installment about the petrol we are currently getting from the pumps of the corner gas station. In this installment is a discussion regarding the long argued possibility of engine damage due to the use of unleaded gasoline in engines.
As the Environmental Protection Agency (EPA) mandated the reduction in lead content gasoline over the last ten years one of the major concerns among the consumers was the possibility of engine damage, specifically to the valves and valve seats. During this time the EPA continued to claim that no meaningful damage would be done by the use of unleaded fuels in engines designed for leaded gas. However the truck, farm, RV, car collector and motor sports enthusiasts claimed otherwise. The EPA continued to claim no damage despite the fact the EPA had never conduct hard tests on the affects of the use of unleaded petrol.
In 1985 Congress forced the hand of the EPA by passing the Food Security Act, within this act was a provision mandating that the EPA to test engine durability using unleaded fuels in engines designed for use of leaded gas and engines equipped with hardened valve seats (designed for use of unleaded fuels). So what did the EPA discover? And why weren't the test results publicized?
The EPA test included eight engines, four were tractor engines, one combine engine, two light-duty farm-truck engines, and one heavy duty truck engine. Quite obviously the four tractor engines are of no interest to the motorsports enthusiasts, nor is combine. The light-duty truck engines are another matter, their use would likely simulate transportation car use. The question here for motorsports nuts is if such use simulates the hard acceleration, high revs sustained in a hard driven sports car, specifically our "Properly" driven Lotuses. The argued answer to this question is "no", that in fact the heavy-duty truck engine used in the EPA test is more demonstrative of the possible results of using unleaded fuel in a "Properly" driven Lotus. Why? Simply put the heavy-duty truck engine is subjected to constant loads, simulating hard accelerations and sustained high revs. It can be argued that the slow reving truck engine does not simulate the high reving sports car engine. This argument is probably valid, however when considering the pounding the valve seats take from big, heavy truck valves compared to lighter and smaller sports car valves the two are probably more similar than to any of the other engines used in the EPA testing.
The heavy duty truck engine used in the EPA test was a GM 454 equipped with hardened valve seats, designed to be used with unleaded fuels with little or no valve recession. GM however until recently recommended the use of leaded fuels with this engine in RV's and trucks. Prior to testing the engines were completely rebuilt to stock specification and then run in for 12.5 hours using leaded gas with 1.2 grams of lead per gallon. After the break in was complete new OEM heads were fitted for the actual testing. The test was conducted for a total 200 hours on each engine.
The fuels used in the test were a commercially available unleaded regular gas, this was the base blend. To this base blend tetraethyl lead was added to create blends with 0.1 grams/gallon and 1.2 gram/gallon. The test simulated road load conditions that reflected the use of a relative large truck at highway speeds, lower speeds of urban type driving and near maximum speed and load conditions. These testing cycles were repeated 16 hours per day for a total of 144 hours. At the end of the 144 hours, a steady state mode of 100hp at 3000 rpm for 16 hours per day was used until the 200 hour mark was reached.
At the end of each 16 hour cycle the valve train was measured for wear and adjustments were made to valve clearances in order to prevent failure. The cooling system was maintained at 230 degrees F. Ambient air temperature was held at 85 +/- 5 degrees. Humidity was not controlled but monitored throughout the testing. Engine oil was changed every 100 hours and oil was added to keep the sump level up as needed. At each oil change the oil was analyzed for the presence of wear metals.
After testing the engine with one type of fuel, the heads were removed and inspected and new fresh heads were fitted for the next cycle of testing. Testing of this sort on a dyno is considered quite aggressive, reflecting full bore acceleration and heavy hill climbing, with high coolant temps. In real life constant hill climbing and constant hard acceleration would not be done, afterall there to every hill there's a down and to every straight there's a corner at the end.
The results were eye opening. When the 454 was tested for 200 hours as described above, using leaded 1.2 gram/gallon petrol there was no valve recession, none of the valve seats showed recession in excess of 0.007 inches. Not surprising. 1.2 gram/gallon of lead protected the valves, but this level of leaded gas is no longer available from the pump.
The results of the 200 hour test using unleaded gas were quite dramatic. The 454 with induction harden valve seats showed valve recession measuring from 0.007 to a high of 0.032 inch. Consider that the 200 hour test would be the equivalent of about 10,000 miles at a constant speed of 50mph. Consider that catastrophic engine failure can take place when the valves recess about 0.060 to 0.080 inch. Thus one can concluded that at the rate of 0.032 inch per 200 hours, that at 20,000 to 25,000 miles of driving the engine could fail! It should be stressed that the engine was fitted with harden valve seats, designed to burn unleaded fuel. What would happen to an engine not designed for unleaded? I don't to imagine...
A second test was run with unleaded gas. The heads were fitted with hard valve-seat inserts such as those that could be installed in an engine to convert it to operate on unleaded gas. At the end of the 200 hours of testing the valve seat recession was fairly consistent across all valves with a maximum of 0.017 inch.
A 454 was tested using the induction harden valve seats using low-lead, 0.10 gm/gal, leaded pump gas. At the end of 200 hours of testing there was essentially no recession of the exhaust valve seats.
Of final interest a GM 292 six cylinder engine with cast iron (soft) valve seats (designed for leaded gas) was tested using unleaded fuel. After only 88 hours the test had to be discontinued when one of the valve seats had recessed 0.099 inch!
So what can be concluded? Although the EPA refrained from drawing any conclusions from their testing the results speck for themselves. We can draw the following conclusions:
1) Engines designed for leaded gasoline are at RISK when burning unleaded fuels.
2) Engines fitted with either induction-hardened seats or hardened valve seat inserts can survive on a diet of low-lead 0.10 gm/gal gasoline.
3) Engines fitted with induction-hardened valve seats can suffer damage when subjected to unleaded gasoline.
So, what do we as Lotus owners do? The solution is to use harden valve seats and stainless steel valve so you can burn unleaded fuels. For added protection use a lead substitute additive, Redline, Unocal, etc. An octane booster may help to gain a few octane points, when added to unleaded pump premium.
Orwell wrote in his famous novel, "Animal Farm," that all animals were equal... but some animals are more equal than others... This statement has never been more true for the petrol that we pump into our cars at our favorite service station.
Too often I've heard some Lotus enthusiast claim that one particular brand of petrol is better for his Lotus than some other brand. Thus they claim that the Orwellian statement about equality holds for their particular brand of gas. "Don't use the cheap stuff", they shout. "Don't even think about XYZ brand... It does some unpronounceable thing to your engine." Meanwhile Joe Lotus-nut is ranting that all pumped gasolines are equal (beyond the octane)... "Just read the octane numbers... that's all", says he.
So what's really the truth?
Let's explore... Let's take a look at say the area in which I live... Most the petrol around the Palo Alto - Santa Clara basin is delivered to the stations via tanker trucks that fill up their trailers at the San Jose Fuel terminal. Now, anyone visiting this terminal will note that the trucks coming and going represent all sorts of brands of petrol companies, Arco, Mobil, Shell, Chevron, Unocal, Texaco, Exxon, and the independents. And yet as you stare into the terminal it becomes extremely evident that if you assume each brand has at least 3 types of gasoline, that there are not enough storage tanks to supply these multitudes of various brands and types. What's going on?
Then too not all the major petrol makers have refineries within a reasonable distance of the market they are servicing. Here in the Bay Area Unocal, Exxon, Shell, Chevron and Tasco have refineries in the Benicia, Martinez and Richmond areas. From these refineries the refined fuels are piped to one of the two terminals for shipment to a variety of Northern California locations. These terminals are located in Oakland and in Concord.
The San Jose sub-terminal receives its supply of fuel from the Concord terminal. Interestingly there is only ONE 10" pipe that runs from Concord to San Jose. The fuels are "shipped" in "batches". Therefore in one period unleaded fuel maybe travelling through the pipeline and later down the same line diesel fuel maybe be shipped. This means that there maybe some mixing of uncompatible fuels. This mixture is shunted off and return to the refinery to be reprocessed.
Roughly 3 million gallons are shipped through this pipe each day. Once out of the pipe the batches of fuel are separated into various holding tanks. These fuels are then picked up by each company's tanker trucks. As the trucks are filled the additives that make one company's product different from another are added. But, because there are more companies than holding tanks, some of the fuels are stored in common tanks. There is no way that anyone can determine if the gasoline being loaded in a say Shell truck was actually refined at the Shell refinery. This goes the same for any brand of petrol.
So, quite possibly the next time you pull up to a Unocal pump you could be filling your tank up with gasoline refined at an Exxon refinery.
The question remains... Are all gasolines equal or some more equal than others?